Tip
Tap
a student's name
below to see what they need help with20 questions
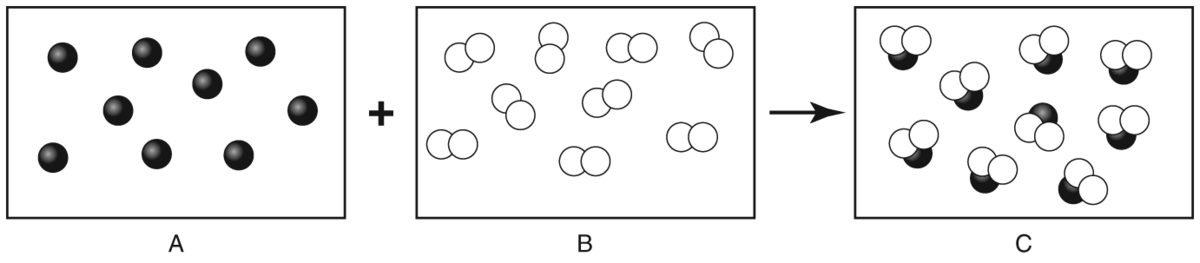
Q.Reactant A and reactant B undergo a chemical reaction to form product C. What is reactant B?
1
60 sec
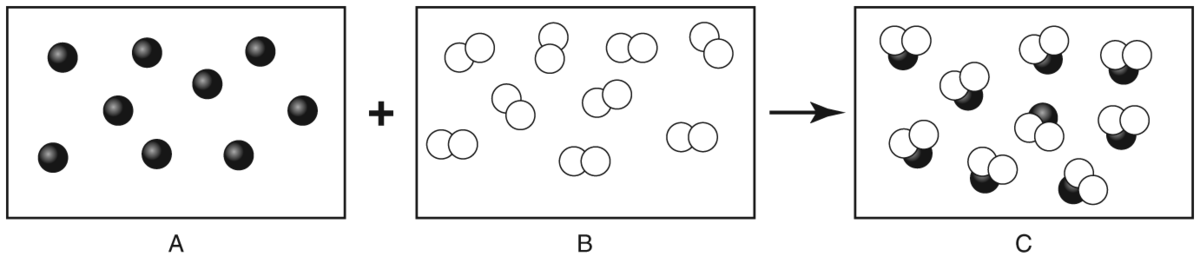
Q.The diagram below shows what happens during the formation of a compound. The diagram shows two substances joining together to form a third substance. Which one is a compound?
2
60 sec
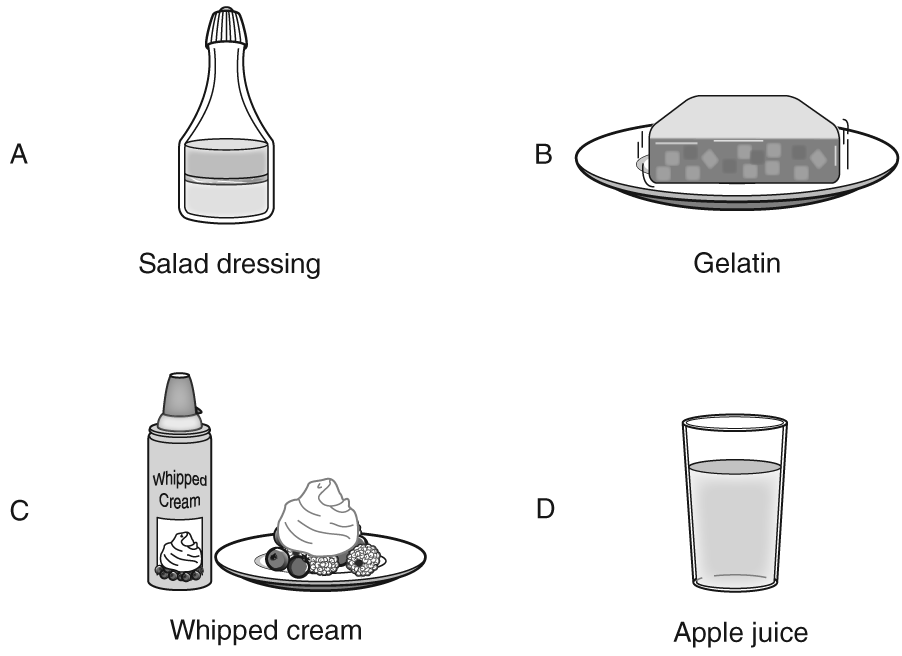
Q.The four items shown below were served at a dinner at Kim’s house. Each of the items is a mixture. Which one is a solution?
3
60 sec
Q.What type of substance is always made up of a single type of atom?
4
60 sec
Q.Assume water and other substances are mixed to form a solution, a suspension, and a colloid. Which type of mixture contains the largest particles?
5
60 sec
Q.Muddy water is an example of what type of mixture?
6
60 sec
Q.What is true of all mixtures?
7
60 sec
Q.What kind of a heterogeneous mixture is the represented in the picture?
8
60 sec
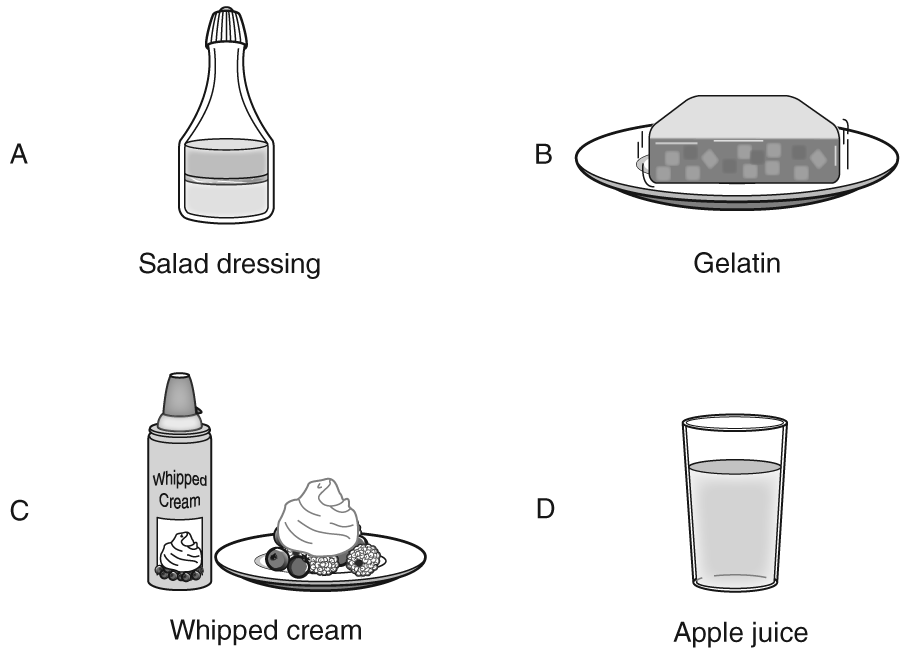
Q.The four items below were part of a dinner. Each item is a mixture.
Which of these mixtures is a suspension?
9
60 sec
Q.How could you break down the compound calcium carbonate into the elements that make it up?
10
60 sec
Q.A glass of Kool-Aid is an example of a
11
60 sec
Q.Which of these common substances is a homogeneous mixture?
12
60 sec
Q.Solution is another name for what type of matter?
13
60 sec
Q.A substance contains an arrangement of different types of atoms joined together by chemical bonds. Which of the following classes of substances could this describe?
14
60 sec
Q.Which of these substances is an example of a solution?
15
60 sec
Q.What is a homogeneous mixture?
16
60 sec
Q.What is the main difference between elements and compounds?
17
60 sec
Q.what is the main difference between homogeneous and heterogeneous mixtures?
18
60 sec
Q.Parts that make a mixture will always keep their original properties
19
60 sec
Q.What kind of a mixture is pizza?
20
60 sec