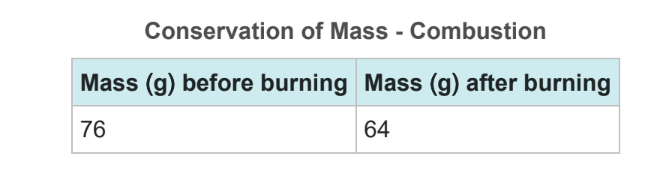Tip
Tap
a student's name
below to see what they need help with20 questions
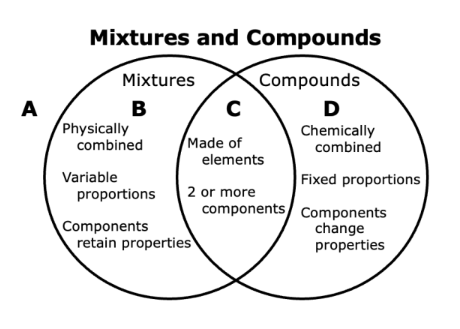
Q.What is the difference between a pure substance and a mixture?
1
Text to speech
120 sec
S8P1a
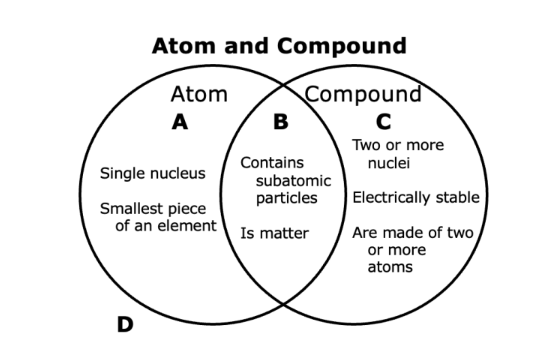
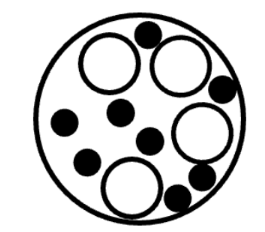
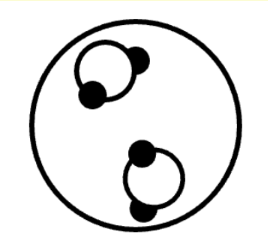
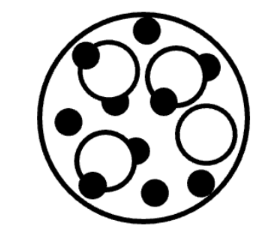
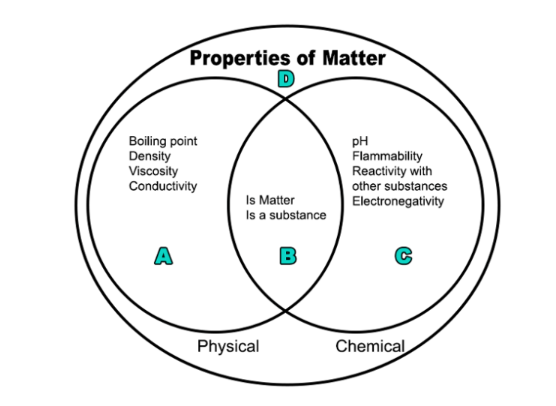
Q.Summarize the Venn diagram.
2
Text to speech
120 sec
S8P1a
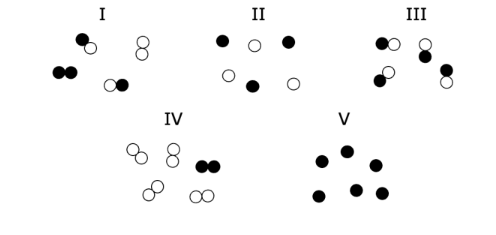
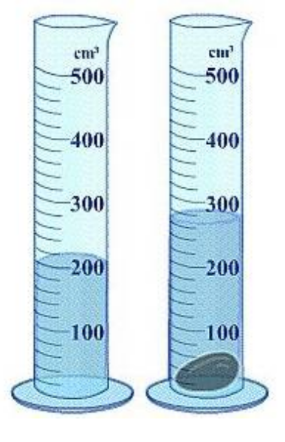
Q.Which image(s) would be considered pure substances?
3
Text to speech
120 sec
S8P1a
Q.Elaborate on how to classify elements and pure compounds.
4
Text to speech
120 sec
S8P1a
Q.To make salt water a heterogeneous mixture, you would have to
5
Text to speech
120 sec
S8P1a
Q.When a substance such as ice melts, its temperature increases. Describe what happens to the arrangement of the water molecules as the temperature increases
6
Text to speech
120 sec
S8P1b
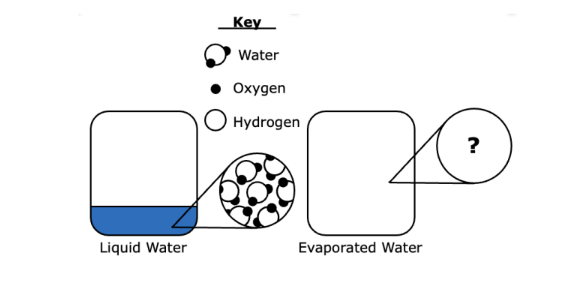
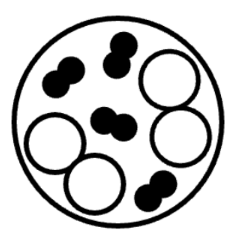
Q.The circle on the left shows a magnified view of a very small portion of liquid water in a closed container. What would the magnified view show after the water evaporates?
7
Text to speech
120 sec
S8P1b
Q.Elaborate on the reason(s) that matter is said to move even as in a solid state.
8
Text to speech
120 sec
S8P1b
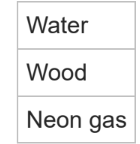
Q.Arrange the substances in the table from the MOST to the LEAST ordered particle arrangement.
9
Text to speech
120 sec
S8P1b
Q.When she dropped a rock into a graduated cylinder of water, Veronica noticed it sank to the bottom. She decided to investigate what it would do if she tossed it into a much larger amount of water. When she tossed it into a lake, the rock sank again. Which statement best explained why this happened?
10
Text to speech
120 sec
S8P1c
Q.A student wants to conduct an investigation that will determine a physical property of an unknown substance. Which of these procedures would best measure a physical property of the unknown sample?
11
Text to speech
120 sec
S8P1c
Q.You measure an unknown substance with litmus paper and determine it to be a strong acid. Where would you place this property in the Venn diagram?
12
Text to speech
120 sec
S8P1c
Q.Atom A has an atomic number of 19 and mass number of 40. Atom B has an atomic number of 20 and a mass number of 40. Which of these is an accurate statement?
13
Text to speech
120 sec
S8P1e
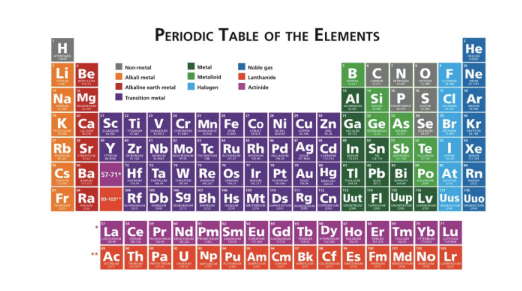
Q.The periodic table is an arrangement of the chemical elements, ordered by atomic number, into families, and by doing so illustrates the periodic properties of the elements. Scan the periodic table from left to right. What trends among the properties of elements are apparent? All BUT ONE could apply.
14
Text to speech
120 sec
S8P1e
Q.Neutrons are found in the nucleus and separate the other particles so that the strong force can hold the nucleus together. Which particles are positively charged and need to be separated so that they don't repel?
15
Text to speech
120 sec
S8P1e
Q.Maria and Carla were hiking when Maria tripped, fell, and sprained her ankle. Carla took out the first-aid kit and unwrapped a cold pack. Carla popped a vial that was inside the cold pack. The pack began to get icy cold and Carla applied the pack to Maria's ankle. What kind of reaction occurred in the cold pack? What evidence supports your answer?
16
Text to speech
120 sec
S8P1d
Q.Ben and Sam were conducting experiments on physical and chemical changes in science class. First they added some salt to water and stirred it up until the salt dissolved. "That's a physical change" Ben said. "And that's a solution which is a mixture", added Sam. Next they added an anti-acid tablet to some water. The water began to fizz and bubble. The beaker got cooler. "That's a physical change too," Ben noted. "No, it's not," responded Sam. Who is correct and where should the contents of the beaker be placed in the Venn diagram?
17
Text to speech
120 sec
S8P1d
Q.glass shattering into pieces
bubblegum being stretched a piece of metal expanding due to heat
These are all examples of
18
Text to speech
120 sec
S8P1d
Q.While studying chemical reactions and conservation of matter, Sarah's class conducted a variety of experiments. Sarah and her lab partner found the mass of a wax candle and placed the candle in a flask. The students lit the candle and let it burn to completion. All that was left was wax and charred candle wick. Per their teacher's instructions, the students found the mass of the products. Their data can be seen in the data table. Formulate a hypothesis the students could test to explain why the experiment did not support the law of conservation of matter.
19
Text to speech
120 sec
S8P1f
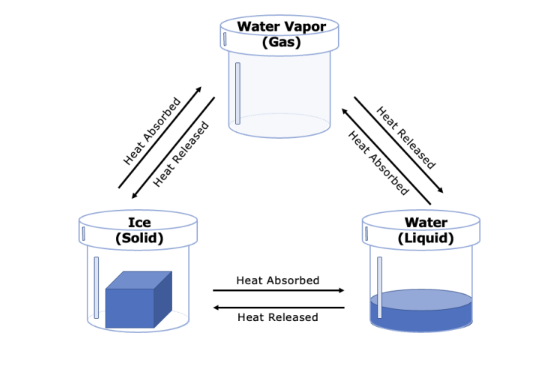
Q.If water with the mass of 15.0 grams is heated into water vapor in a closed system such as a closed glass jar, what is the mass of the gas after the water completely evaporates?
20
Text to speech
120 sec
S8P1f